Thailand FDA Issues Traveller-Friendly Guidelines for Importing ...
The FDA provides clear instructions for bringing various health products – including medicines, dietary supplements, cosmetics, and medical devices – into Thailand. Tourists are encouraged to familiarise themselves with the guidelines to ensure compliance and avoid unexpected delays or issues at customs.
Carrying Health Products into Thailand
Travellers may carry certain health products for personal use, though quantity restrictions and documentation requirements apply:
Medicines: A 30-day supply for personal use is allowed, provided the medicines remain in their original packaging and are clearly labelled. If the product is a controlled substance, it must be accompanied by a prescription or doctor’s letter. Cosmetics: Ready-to-use products are permitted, with a maximum of three pieces per item and a total limit of 15 items. Food Items: Up to 10 kilograms/litres of food for personal consumption is allowed. Medical Devices: Allowed in necessary quantities for personal use, supported by relevant documentation if required.Travellers should be aware that certain items, including narcotics, cannabis and hemp-based products, psychotropic substances, and unregistered or prohibited products, are restricted. Possession of these items may lead to penalties or legal action, as Thailand enforces stringent safety standards for health-related imports. The FDA advises all tourists to verify their health products’ compliance with Thailand’s import regulations before travel.

Importing Health Products into Thailand by Mail
For tourists planning to send health products by international mail, the FDA requires items to be clearly labelled, strictly for personal use, and within permitted quantities:
Food, Cosmetics, and Medical Devices: These items must comply with on-person restrictions, ensuring they meet Thailand’s import laws and contain no restricted ingredients, such as cannabis extracts. Hazardous Substances: While some household-use hazardous substances are allowed, Category I substances require prior notification to the FDA, and Categories II and III are limited to 5 kilograms/litres.Accurate labelling is recommended to ensure smooth processing through customs, minimising the risk of confiscation or delay.
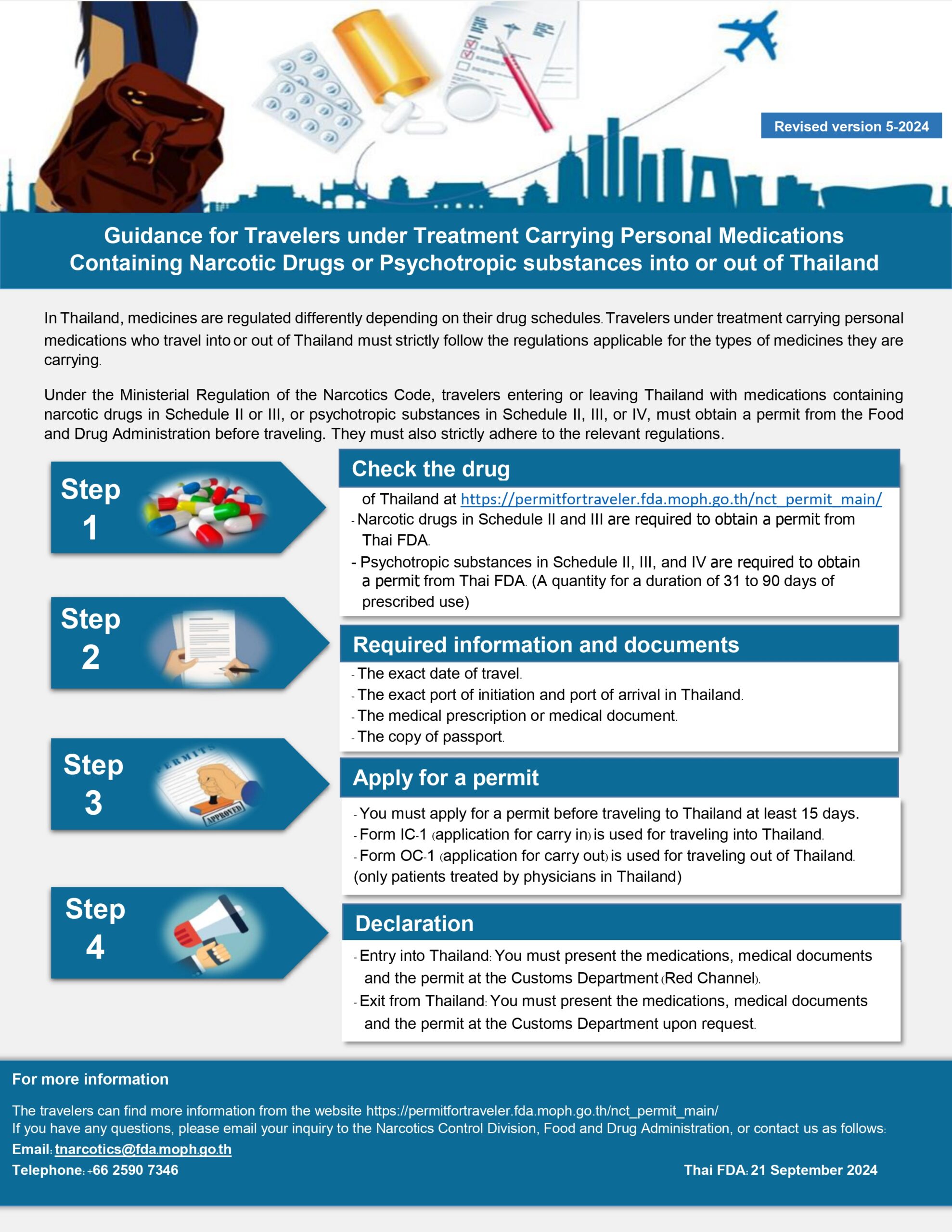
Guidance for Travellers Carrying Personal Medications with Narcotic or Psychotropic Substances

For travellers carrying medications containing narcotic or psychotropic substances, specific compliance measures are required:
Narcotics and Psychotropic Substances: A 30-day supply with a prescription is allowed. For larger amounts (up to 90 days), a permit from the FDA (Form IC-2) is required and must be obtained at least 15 days prior to travel. Permit Requirements: Travellers must present their permit, prescription, and medications to Customs upon entry, using the Red Channel for declaration.The FDA encourages all travellers to confirm that their health products align with Thailand’s import regulations before their trip to ensure a smooth, compliant experience. Comprehensive resources, step-by-step instructions, and relevant forms are available in English on the FDA’s official website.
This effort is part of Thailand’s commitment to maintaining high health standards while welcoming international visitors. By following these guidelines, tourists can enjoy a worry-free travel experience, assured of their compliance with Thailand’s health and safety regulations.
The TAT International Public Relations Division works with traditional and online media channels to promote Thailand as a tourism destination for travellers worldwide.









































